|
The gases are mixable with each other in all proportions. Since the ideal gas law
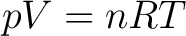 |
(1) |
is valid for any ideal gas, one may think that it's insignificant whether the mole number 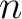 concerns one single gas or several gases. It is true, which can be shown experimentally. concerns one single gas or several gases. It is true, which can be shown experimentally.
Let's think that we mix the volumes 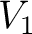 , , 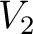 , ..., , ..., 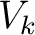 of different gases having an equal pressure of different gases having an equal pressure 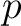 and an equal temperature and an equal temperature 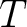 . If one measures the volume . If one measures the volume 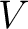 of the mixture in the same pressure and
temperature, one notices that of the mixture in the same pressure and
temperature, one notices that
Each of the gases satisfies an equation
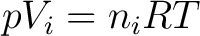 , and thus , and thus
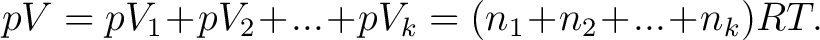 |
(2) |
This is similar as the general equation (1). If we think that the same volume 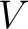 would be filled by any of the gases alone, we had an equation
for each gas; here the pressure would be filled by any of the gases alone, we had an equation
for each gas; here the pressure 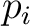 , i.e. , i.e.
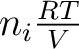 , is called the partial pressure of the gas , is called the partial pressure of the gas 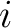 . By (2), we have
Accordingly we have obtained the . By (2), we have
Accordingly we have obtained the
Dalton's law. The pressure of a gas mixture is equal to the sum of the partial pressures of the component gases.
This law was invented by J. Dalton in 1801.
|

