|
The Wien Displacement Law can be used to find the peak wavelength of a blackbody at a given temperature. Planck's radiation law gives us a function of 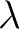 and temperature so we can find the maximum of this function and hence the peak wavelength emitted [1]. and temperature so we can find the maximum of this function and hence the peak wavelength emitted [1].
So for a given T we have
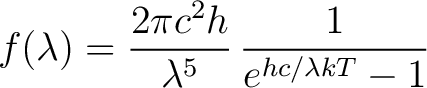 |
(1) |
To find the peak of this function differentiate with respect to 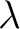 and set it equal to 0 and set it equal to 0
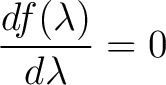 |
(2) |
Use the product rule to carry out this differentiation
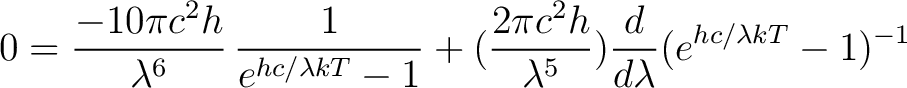 |
(3) |
Next use the chain rule to get
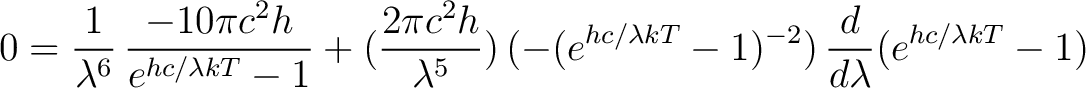 |
(4) |
Apply the chain rule again
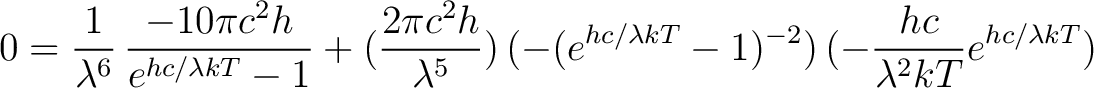 |
(5) |
Multiply both sides by
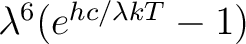
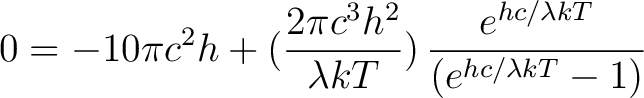 |
(6) |
Pull the e term into the denominator and divide out
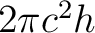 to get to get
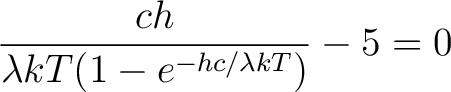 |
(7) |
This leaves us with a transendental function, which must be solved numerically
Set
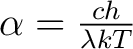 and substitute into above and substitute into above
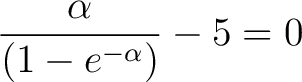 |
(8) |
After solving this equation for 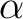 , the result yields Wien's Law , the result yields Wien's Law
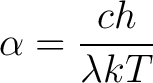 |
(9) |
rearranging
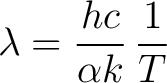 |
(10) |
A simple way to find 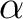 is to use Newton's Method. This can be done by hand or with your favorite numerical program. Some matlab routines have been attached to see how to get is to use Newton's Method. This can be done by hand or with your favorite numerical program. Some matlab routines have been attached to see how to get 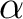 . .
To use Newton's Method we need we rewrite and arrange (8) to get
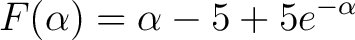 |
(11) |
We also need the first derivative of this so
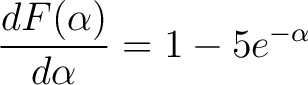 |
(12) |
Then through iteration we can converge on the solution
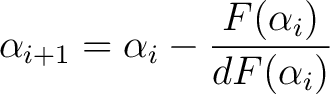 |
(13) |
For our accuracy needs we choose
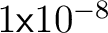 so we stop iterating when so we stop iterating when
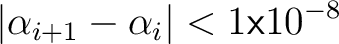 |
(14) |
In matlab you can run WienConstant.m which depends on fWien.m and dfWien.m and will get a value for 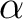 . So we see . So we see
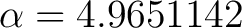 |
(15) |
Plugging this value into (10) and evaluating the other constants yields the Wien Displacement Law, which gives the peak wavelength for a given temperature of a blackbody.
![$\displaystyle \lambda = \frac{2.897 \mathsf{x} 10^{-3} \, [Km]}{T}$ $\displaystyle \lambda = \frac{2.897 \mathsf{x} 10^{-3} \, [Km]}{T}$](http://images.physicslibrary.org/cache/objects/20/l2h/img26.png) |
(16) |
Note that the temperature must be in Kelvin [K] and then 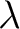 will have units of meters [m]. At different temperatures a blackbody's peak wavelength is displaced, hence the name Wien's Displacement Law. will have units of meters [m]. At different temperatures a blackbody's peak wavelength is displaced, hence the name Wien's Displacement Law.
[1] Krane, K., "Modern Physics." Second Edition. New York, John Wiley & Sons, 1996.
| 
