|
|
|
Main Menu
|
|
Sections
Meta
Talkback
Downloads
Information
|
|
|
|
|
|
mean translational kinetic energy
|
(Definition)
|
|
|
The mean translational kinetic energy is a result from the Maxwell-Boltzmann distribution and is given by
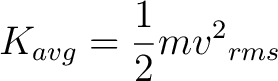 ( 1)
A more useful form of this equation is in terms of temperature. Temperature is brought in through the root mean square speed derived in the kinetic thoery of gases
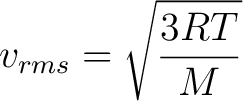 ( 2)
where R is the gas constand
![$\displaystyle R = 8.31 \, [ \frac{J}{mol * K} ] $ $\displaystyle R = 8.31 \, [ \frac{J}{mol * K} ] $](http://images.physicslibrary.org/cache/objects/23/l2h/img3.png) ( 3)
and more to come ...
|
"mean translational kinetic energy" is owned by bloftin.
|
|
| Other names: |
average translational kinetic energy |
Cross-references: speed, square, temperature
There is 1 reference to this object.
This is version 3 of mean translational kinetic energy, born on 2004-11-24, modified 2005-07-27.
Object id is 23, canonical name is MeanTranslationalKineticEnergy.
Accessed 2713 times total.
Classification:
|
|
|
|
|
|
|
|
Pending Errata and Addenda
|
|
|
|
|
|
|
|
|
|
|

