|
Entropy has a long and rich histroy in physics and was developed by many scientists over the years including Clausius, Maxwell, Boltzmann, Planck, Gibbs, Pauling and more recently Shannon and Hawking. The applications of entropy are far and wide ranging from engines to black holes. The concept of entropy was introduced by the German R. J. E. Clausius in 1854.
The classical definition of entropy relates the differential change of entropy to the differential change in heat at a given temperature during a reversible process:
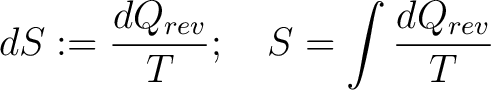 |
(1) |
The belief that entropy is a measure of the disorder of the system is a misconception.
Entropy, this entry is world editable, so feel free to contribute. Note please be courteous and document your changes with comments and discuss through posting.
Beginner
[1] Halliday, D., Resnick, R., Walker, J.: "fundamentals of physics". 5th Edition, John Wiley & Sons, New York, 1997.
Intermediate
[2] Kondepudi, D., Prigogine, I. "Modern Thermodynamics From Heat Engines to Dissipative Structures" John Wiley & Sons, Chichester, 1998.
[3] Kittel, C., Kroemer, H. "Thermal Physics" Second Edition. W.H. Freeman and Company, New York, 1980.
Advanced
[4] Greven, A., Keller, G., Warncke, G. "Entropy" Princeton University Press, New Jersey, 2003.
|

